Measuring range & accuracy
Measurement range: Ca 300 - 498 mg/l (ppm)
Measurement range: Mg 820 - 1800 mg/l (ppm)
Accuracy: Ca 4 mg/l
Accuracy: Mg 20 mg/l
About calcium and magnesium
Along with sodium, potassium, chloride and sulphate, calcium (Ca2+) and magnesium (Mg2+) are two key components of natural seawater. Magnesium and, above all, calcium are important growth factors for organisms which form calcareous skeletons, such as hard coral andcoralline algae - both elements form the basic substance of the calcareous skeleton. Furthermore, magnesium and calcium are involved in numerous biochemical processes. In saltwater applications, the reduction in the magnesium or calcium concentration caused partly by cellular metabolism and partly by skeleton formation means it is necessary to test the concentration levels on a regular basis and, if required, add more of one or both ions. This makes it possible to guarantee optimum, near-natural living conditions for all organisms and to prevent long-term damage.
In natural seawater, the calcium concentration is 400-410 mg/l (ppm) and the magnesium concentration is 1280-1320 mg/l (ppm). They also have a fixed ratio of 1 : 3.25 to one another. Due to the chemical and biochemical interdependencies between calcium and magnesium, you should also aim to achieve this concentration ratio in saltwater tanks.
Package contents
- 35 ml/1.38 fl.oz. of reagent A
- 9 g/0.32 oz. of reagent B
- 50 ml/1.69 fl.oz. of reagent C
- 40 ml/1.35 fl.oz. of reagent D
- 2 glass cuvettes 10 ml
- 1 dosing syringe 5 ml
- 3 dosing syringes 1 ml with dropper tips
- 1 measuring spoon
-1 instruction for use
Instructions for use
Note: The calcium concentration is determined first, and then the magnesium concentration is determined using the same sample. To avoid cross-contamination, the dosing syringes with the proper tips must only be used again for the same reagents!
Dip the syringe into the liquid before drawing up the syringe. Readings on dosing syringes are always taken at the plunger even if there is air between the plunger and the liquid (caused by the empty volume of the dropper tip). The air bubble will not affect the tes tresult.
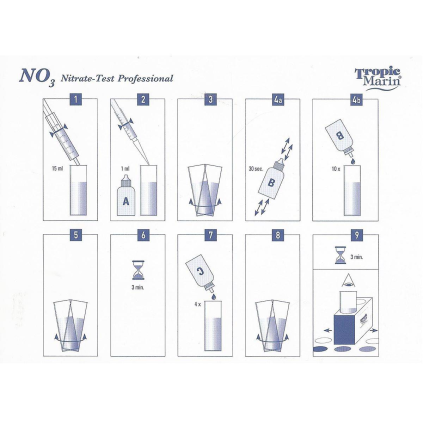
Determining the calcium level:
1: Shake the bottles before use!
2: Rinse out both glass cuvettes with tap water and then several times with aquarium water.
3: Fill each glass cuvette with exactly 5 ml of aquarium water using the dosing syringe. Put one of the two water samples aside as a reference.
4: Place a clean dropper tip onto the 1 ml dosing syringe with red lettering and draw out reagent A up to the 20 marking on the syringe (corresponds to 0.5 ml). Empty the entire amount into the cuvette with the analysis sample.
5: Close the glass cuvette using the stopper and briefly swirl the solution.
6: Then mix reagent B (powder) with the measuring spoon and add 1 level measuring spoon of reagent B to the glass cuvette containing the analysis sample. Swirl the cuvette carefully until the powder has dissolved. The water sample will turn light-blue.
7:Place another clean dropper tip onto the 1 ml dosing syringe with black lettering and draw out 1 ml of reagent C.
8: Now add reagent C from the syringe to the analysis sample drop by drop until the light-blue solution becomes colourless*. Swirl the cuvette after each new drop. To assist in detecting the colour change, take the comparison sample (the second cuvette you prepared at point 3) on a white surface and look into both cuvettes from above when they are sitting side by side. You can stop adding drops when the colour change is complete and there is no difference between the analysis sample and the comparison sample.
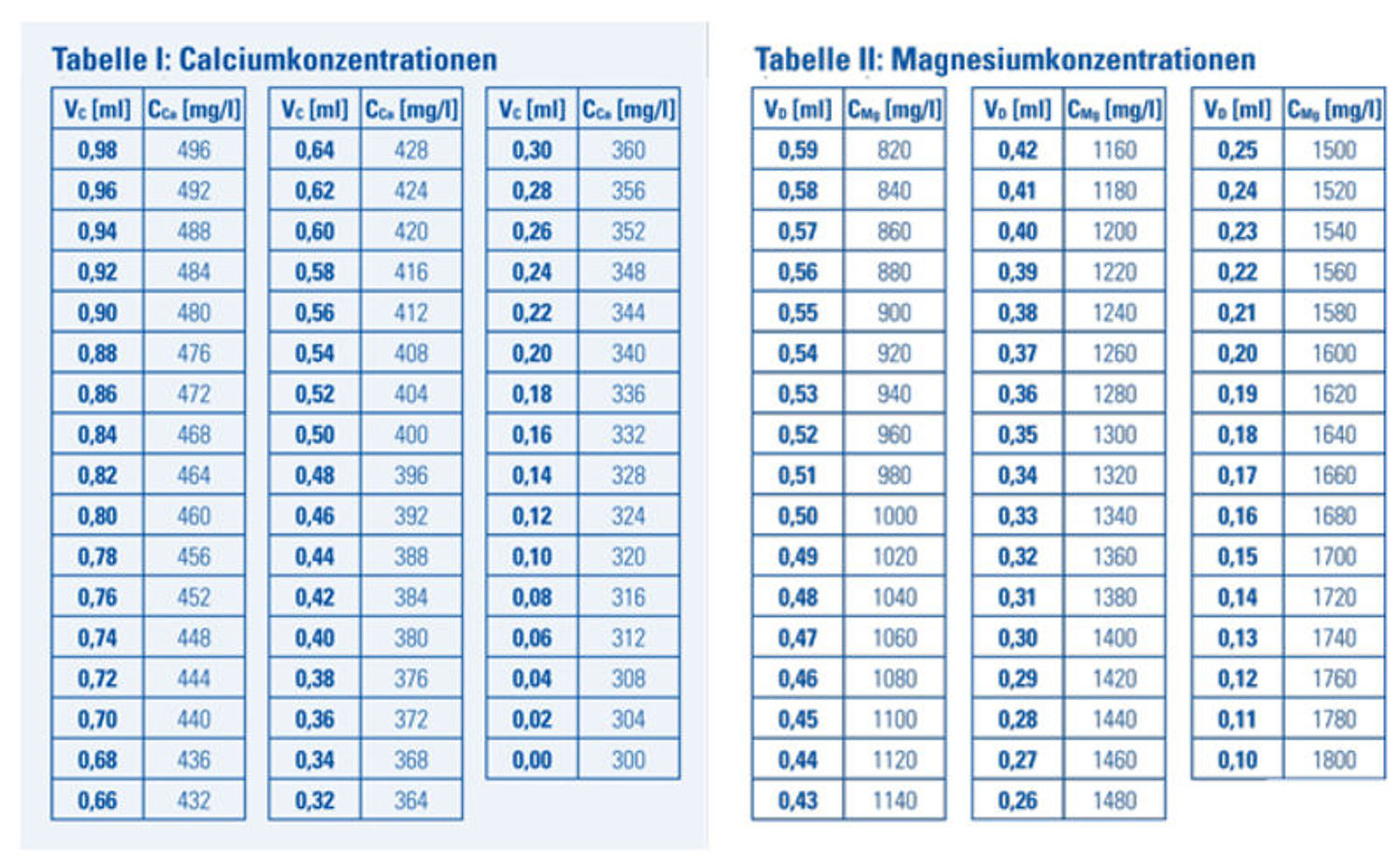
9: The residual volume of reagent C in the syringe shows the calcium concentration CCa in mg/l (ppm) that can be determinedusing table I.
Example: If the lower side of the syringe plunger is at 0.46 ml following titration, then the residual volume (VC) of reagent C is 0.46 ml. The calcium concentration in the sample is: Ca = 392 mg/l.
10: The residual volume of reagent C in the syringe can be put back into bottle C.
*Note: If the solution becomes colourless after adding only two drops of reagent C, we recommend repeating the determination process with a lower sample volume of 4 ml instead of 5 ml. Carry out the test in accordance with the specifications, however, at point 3, use a sample of 4 ml per cuvette. You must multiply the calcium concentration value obtained from the table at the end of the determination process by the factor 1.25 to establish the true calcium concentration in your sample.
Example:
Sample volume in ml: 4 ml instead of 5 ml
Calcium concentration reading: 440 mg/l (ppm)
Actual calcium concentration: CCa = [1.25 x 440 mg/l] = 550 mg/l (ppm)
Determining the magnesium level (must follow the calciumtest with the same sample):
1: Place the third clean dropper tip onto the 1 ml dosing syringe with the green plunger and draw out 1 ml of reagent D.
2: Add approx. 0.4 ml of reagent D to the water sample. The water sample will turn light-blue again.
3: Now add the remaining reagent D from the syringe to the water sample drop by drop until the light-blue solution becomes colourless**. Swirl the cuvette after each new drop. To assist in detecting the colour change, take the comparison sample (the second cuvette) on a white surface again and look into both cuvettes from above when they are sitting side by side.
4:The residual volume of reagent D in the syringe shows the magnesium concentration CMg in mg/l (ppm) that can bedetermined using table II.Example: If the lower side of the syringe plunger is at 0.35 ml following titration, then the residual volume (VD) of reagent C is 0.35 ml. The magnesium concentration in the sample is: Mg = 1300 mg/l (ppm). If you have a 4 ml sample, multiply the table value by 1.25 to establish the correct magnesium concentration._
5: The residual volume of reagent D in the syringe can be put back into bottle D. Rinse out the glass cuvettes, dosing syringes and dropper tips thoroughly with tap water and allow to dry before using them again.
** Note: If the colour change from light-blue to colourless is difficult to detect, we recommend to carry out the measurement under a bright light source which is similar to daylight.
Shelf life and storage:
6 months after opening. Store in a cool, dark place.
Table I: Table II:
Calcium Concentrations Magnesium Concentrations
Reagent left in syringe VC [ml] Reagent left in syringe VD [ml]
Calcium Concentrations CCa in mg/l / ppm Magnesium Concentrations CMg in mg/l / ppm












Legg igjen en anmeldelse